New Imaging Strategies
Angiogenesis Imaging
We are developing targeted ultrasound contrast agents to detect neovascularization. We have chosen to target angiogenic receptors located exclusively on the endothelium, with the premise that detection of these receptors by imaging techniques will permit identification and localization of angiogenesis and monitoring of therapies. While this is being applied to myocardial ischemia, such a strategy can be employed for detecting angiogenesis in other settings, such as solid tissue malignancies. This work is performed in collaboration with Ruth Modzelewski, PhD.
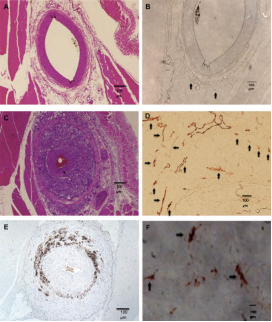
Rabbit femoral artery sections before (upper panels) and 6 weeks after (middle and lower panels) balloon injury. Adapted from Moguillansky D,…, Villanueva FS. Quantification of plaque neovascularization using contrast ultrasound: a histologic validation. Eur Heart J 2010:32.
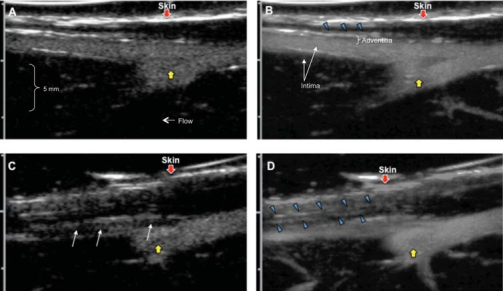
Contrast ultrasound of the rabbit femoral arteries before (A, B) and 6 weeks after (C, D) balloon injury. Adapted from Moguillansky D,…, Villanueva FS. Quantification of plaque neovascularization using contrast ultrasound: a histologic validation. Eur Heart J 2010:32.
Ischemic Memory Imaging
We are addressing the clinical dilemma of making the diagnosis of acute myocardial ischemia in patients presenting to the emergency room with chest pain of unclear etiology. True myocardial ischemia (with or without infarction) is associated with endothelial overexpression of leukocyte adhesion molecules, some of which occurs immediately after onset of ischemia, and which persist for hours thereafter, even after resolution of ischemia. This represents the “ischemic memory” of the myocardial microvasculature, detection of which would confirm the presence and extent of recent ischemia and hence assist in the diagnosis and risk stratification of acute coronary syndromes. This work is performed in collaboration with William R. Wagner, MD of the McGowan Institute of Regenerative Medicine.
Vascular endothelial leukocyte adhesion molecules, such as E-selectin, are acutely upregulated in myocardial ischemia/reperfusion and are thus ‘‘ischemic memory’’ biomarkers for recent cardiac ischemia. Contrast enhanced ultrasound imaging using microbubbles targeted to E-selectin can be used for ischemic memory imaging. Adapted from Leng X, …, Villanueva FS. Ultrasound detection of myocardial ischemic memory using an E-selectin targeting peptide amenable to human application. J Molecular Imaging 2014;13.
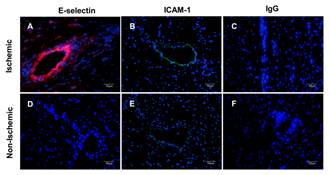
Immunofluorescent staining of post-ischemic (A-C) and nonischemic (D-F) myocardium 4 hours after reperfusion. Nuclei were stained blue with 4',6-diamidino-2-phenylindole, E-selectin was stained red with CY3, and ICAM-1 was stained green with FITC. Non-specific IgG was a control stain. Adapted from Leng X, …, Villanueva FS. Ultrasound detection of myocardial ischemic memory using an E-selectin targeting peptide amenable to human application. J Molecular Imaging 2014;13.
Detection And Molecular Characterization Of Vulnerable Atherosclerotic Plaque
We are pursuing several research directions on the general topic of atherosclerotic plaque imaging. Atherosclerosis is a systemic disease. In patients with coronary artery disease, atherosclerotic plaques and vessel wall disease can be present throughout the coronary tree and be angiographically undetectable. Not all plaques are destined to rupture and lead to acute coronary syndromes. Indeed, most plaques responsible for acute myocardial infarction are those which were not angiographically critical on prior cardiac catheterization. Hence the clinical problem we are trying to address is how to risk stratify atherosclerotic plaques, such that we can predict which are stable vs. which are “vulnerable” to instability and rupture. We are developing imaging strategies to better characterize and identify plaque features associated with vulnerability to rupture, including plaque neovascularization. This work is performed in collaboration with Dr. Wagner.
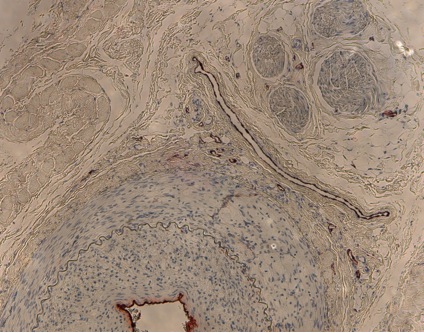
Above: Sample histologic image of atherosclerotic model. Source: Center-generated image.
Stem Cell Tracking
Stem cells of various origins, delivered systemically by vascular injection or locally by direct tissue injection, have shown promise as reparative therapy for infarcted myocardium. Progress in developing this therapeutic modality is hampered in part by a lack of methods to image the stem cells and their fate in vivo. We are developing microbubbles for the purpose of stem cell tracking. The goal is to render stem cells acoustically active prior to delivery, so that after injection, they can be detected in vivo using ultrasound scanning.
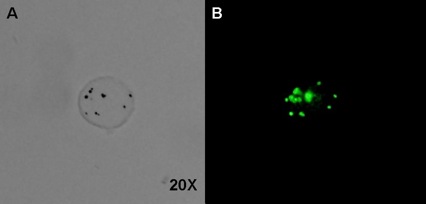
Above: A single mesenchymal stem cell incubated with polymer contrast agent microbubbles and observed under brightfield (A) and fluorescent light (B). The microbubbles, labeled with a green-fluorescing Bodipy dye, are identifiable as dark spots in the brightfield image, and green spots in the fluorescent image. Source: Center-generated image.
Molecular Imaging And Quantification Of Pancreatic Islet Cell Mass
Diabetes mellitus is a growing public health problem in the Western world. The pathogenesis is complex and incompletely understood, but involves a combination of peripheral tissue insensitivity to insulin (as with adult onset diabetes), with or without an absolute decrease in insulin secretion due to failure of the insulin-secreting beta cells of the pancreas (adult- and juvenile-onset diabetes). There appears to be a decrease in islet cell mass associated with diabetes, and the magnitude and rate of decrement may have prognostic implications, particularly in Type 1 (juvenile-onset) diabetes. Unfortunately, there are no clinically reliable in vivo methods for quantifying islet cell mass, and efforts expended by others in developing methods for imaging the pancreas are technically complex (MRI), involve radiation (PET), and have met with limited success. We are currently exploring ultrasonic methods for visualizing and quantify beta cell mass in the islets of Langerhans of the pancreas (collaboration with George Gittes, MD, Children’s Hospital of Pittsburgh of UPMC).
Cardiac Electrophysiology Mapping Using Contrast Agents – NIH Pioneer Award
There are currently no in vivo methods for mapping myocardial electrical conduction in three dimensions and in real time. This limits our ability to understand the mechanisms of arrhythmias and methods to treat them. We are developing an approach to mapping action potential propagation through the heart using contrast agents and ultrasound. We are developing microbubbles that are responsive to electromagnetic energy in ways that are acoustically unique. This work is a collaboration with Barry London, MD, who received an NIH Pioneer Award to pursue this work.
Nonlinear IVUS
Contrast-enhanced intravascular ultrasound imaging is a promising tool for the characterization of coronary vasa vasorum proliferation, which has been identified as a marker of, and possible etiologic factor in, the development of high-risk atherosclerotic plaques. Resonance-based nonlinear detection methods have required the development of prototype catheters which are not commercially available, thus limiting clinical translation. Hence, we have been investigating the performances of a radial modulation imaging approach (a dual frequency approach) using simulations, implemented it on a clinical 20-MHz rotating catheter, and tested it in a tissue-mimicking flow phantom perfused with lipid-encapsulated microbubbles (MBs). Using this imaging strategy, 200-μm diameter cellulose tubing perfused with MBs could be resolved while surrounding tissue scattering was suppressed. These results raise promise for the detection of coronary vasa vasorum and may ultimately facilitate the detection of plaque at risk for rupture.
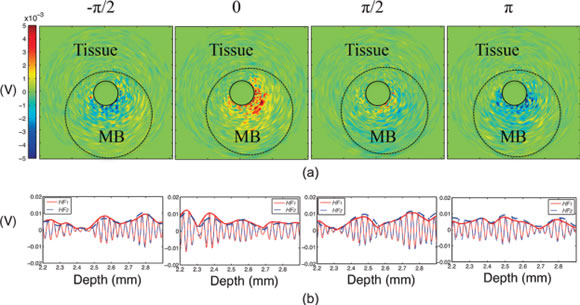
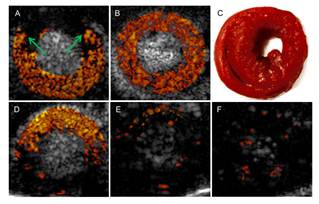
Nonlinear intravascular ultrasound imaging with microbubble based contrast agents. Adapted from Yu F, Villanueva FS, Chen X. Radial modulation contrast imaging using a 20-MHz single-element intravascular ultrasound catheter. IEEE Trans Ultrason Ferroelect Freq Control 2014;61.
Other Ultrasound-Based Molecular Imaging Strategies Not Utilizing Microbubbles
Activities of the Center are not limited exclusively to projects involving the use of microbubble contrast agents. Other acoustic principles which lend themselves to molecular imaging, such as photoacoustics, will be developed by newly recruited faculty. This application involves targeted particles, such as gold nanorods, that generate ultrasound waves when stimulated with light. Such particles can be submicron in size (hence exiting the microvasculature) and have the potential to permit extravascular molecular imaging.


